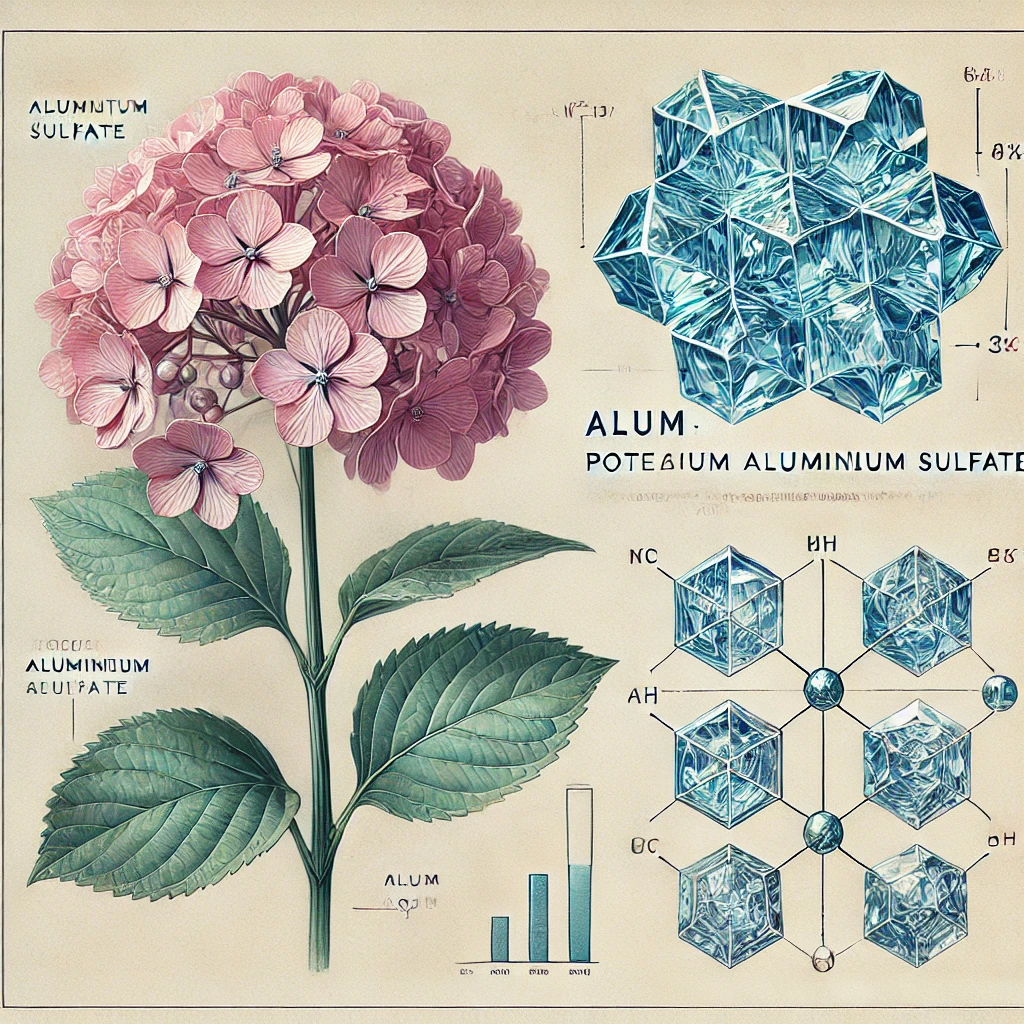
Alum (Potassium Aluminium Sulphate)
Synonyms: Potash Alum, Potassium Alum
Chemical Formula: KAl(SO₄)₂·12H₂O
Common Sources: Natural minerals (Alunite, Bauxite), Industrial synthesis
Botanical and Historical Context
Alum is not a plant-derived substance but a naturally occurring mineral salt historically used in horticulture, medicine, and dyeing. It has been utilized for centuries as a soil amendment, astringent, and mordant in fabric dyeing.
Occurrence in Nature
Alum is commonly extracted from alunite (KAl₃(SO₄)₂(OH)₆), a sulphate mineral found in volcanic and sedimentary rock formations. It can also be derived from bauxite, a primary ore of aluminum.
Uses in Botany and Gardening
- Soil Acidifier: Alum can be used to lower soil pH, making it beneficial for acid-loving plants like hydrangeas, azaleas, and blueberries.
- Color Enhancement in Hydrangeas: The application of alum-based solutions can shift hydrangea blooms from pink to blue by increasing aluminum availability in acidic soil.
- Preservative in Botanical Preparations: Historically used to preserve plant specimens and herbal extracts.
Chemical Properties
- Solubility: Highly soluble in water, forming an acidic solution.
- Astringent Effect: Causes tissue contraction, used medicinally and in tanning leather.
- Antiseptic Qualities: Has mild antimicrobial properties.
Toxicity & Safety Considerations
- Alum is generally safe in small quantities but can be irritating to skin and mucous membranes.
- Overuse in soil can lead to excessive aluminum accumulation, which may be toxic to plants.
- Not to be confused with ammonium alum or sodium alum, which have different applications and properties.